Introduction: Reduced-intensity conditioning (RIC) allogeneic stem cell transplant (allo-SCT) remains a curative option for elderly and/or unfit patients with hematologic malignancies. Fludarabine-containing regimens make up the cornerstone of RIC regimens, however, during the nation-wide fludarabine shortage in 2022, many institutions used fludarabine-free RIC regimens out of necessity though data was extremely limited. Clofarabine is a second-generation purine analog with more potent anti-leukemic activity and a different adverse effect profile compared to fludarabine. We report our experience with clofarabine-based RIC conditioning for adult patients with non-Hodgkin's lymphoma (NHL) and myeloid malignancies.
Methods: In this retrospective single-center matched cohort study at Stanford Health Care, we compared adult RIC allo-SCT patients receiving clofarabine and melphalan (CloMel) with a matched cohort receiving fludarabine and melphalan (FluMel) from January 1, 2022 to December 31, 2022. CloMel consisted of clofarabine 30 mg/m 2 on days -6 to -2 followed by melphalan 140 mg/m 2 on day -1, while FluMel consisted of fludarabine 30 mg/m 2 on days -5 to -2 followed by melphalan 140 mg/m 2 on day -1. Clofarabine dose adjustments were made in patients with renal dysfunction according to the following institutional guidelines based on creatinine clearance (CrCl): 22.5 mg/m 2 for CrCl 60 to 79 mL/min, 15 mg/m 2 for CrCl 30 to 59, and avoiding clofarabine for CrCl less than 30 mL/min. Dose modifications for fludarabine and melphalan due to renal dysfunction were at the discretion of the care team. Patients received graft versus host disease (GvHD) prophylaxis with tacrolimus and methotrexate. Efficacy and safety outcomes were assessed. Data were analyzed using Mann-Whitney U and chi-square tests.
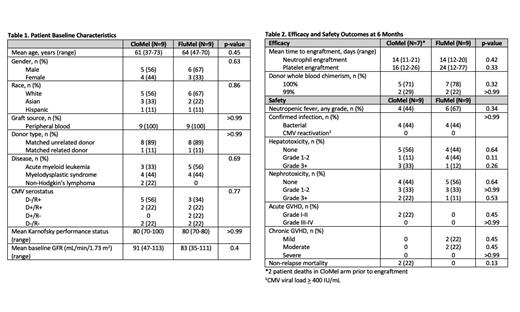
Results: We identified 9 matched pairs for a total of 18 patients. Most patients received an allo-SCT for a myeloid malignancy (8 patients with AML, 8 patients with MDS, and 2 patients with NHL). All patients received a peripheral blood stem cell transplant, with 89% receiving a matched unrelated donor and 11% receiving a matched related donor transplant. Baseline characteristics were balanced between groups. There was no difference in time to neutrophil engraftment (mean 14 vs 14 days; p=0.42), time to platelet engraftment (mean 16 vs 24 days; p=0.33), or 100% donor whole blood chimerism (71% vs 78%; p=0.32) at 6 months in the CloMel and FluMel groups, respectively. No statistically significant difference was observed for the number of patients who experienced acute (2 vs 0; p=0.13) and chronic GvHD (0 vs 4, p=0.09) at 6 months between groups.
At 6 months, 2 patients in the CloMel group and no patients in the FluMel group experienced early non-relapse mortality (p=0.13). Both patients who died experienced acute renal failure secondary to conditioning chemotherapy with a median onset of renal dysfunction 6 days after initiation of clofarabine, followed by sepsis and multi-organ failure. Both patients required initiation of external renal replacement therapy and ultimately passed 12 days and 29 days post-transplant, prior to engraftment. Notably, both patients had a history of kidney dysfunction prior to transplant, one patient with chronic kidney disease (CKD) stage 3 and the other patient with lymphomatous involvement of the kidneys, and received appropriate clofarabine renal dose adjustments per institutional standards.
Conclusion: CloMel demonstrated similar efficacy outcomes at 6 months and represents a feasible alternative to FluMel as a RIC regimen. However, given the notable occurrence of renal failure and early death in the CloMel arm, careful consideration and particular caution in administering to patients with a prior history of renal disease is recommended.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
This study will be discussing the off-label use of clofarabine as part of a conditioning regimen for allogeneic stem cell transplantation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal